Casting is a process for producing alloys of the desired composition and also for specific shapes. These can be either net shapes, as in investment (lost-wax) casting, or stock materials, i.e., ingots, that can be further processed to modify the shape, structure, and properties.
In addition to shaping the metal, the way in which the metal cools substantially affects the microstructure of the metal alloy and, therefore, the properties of the jewelry. Casting should be carefully controlled to ensure high-quality aesthetics and properties.
Quite simply, casting involves pouring molten metal into a mold. As the metal cools in the mold, it solidifies. The casting conditions and the shape of the mold are carefully controlled to ensure a high-quality casting (excellent surface finish and few defects throughout the material) and excellent mechanical properties of the final piece – e.g. strength, hardness, and toughness.
Details of casting
The overwhelming majority of casting tends to be static casting, although techniques such as continuous casting have many advantages for large-scale production.
Static casting usually involves melting by gas heating, oil-fired furnaces, electric resistance heating, or induction heating; the latter ensures maximum stirring of the alloy constituents.
For gold and silver, crucibles are typically clay-graphite, or graphite (fireclay for nickel-white golds as nickel will react with graphite), and casting will usually be into dressed iron molds or water-cooled copper molds.
For platinum and palladium, zirconia or zirconia-washed silica crucibles are preferably used. That said, many casters of palladium use silica crucibles without too much problem, provided temperatures are kept to a minimum and reducing atmospheres are avoided.
Science of casting
Atoms in a liquid are mobile and free to move amongst themselves. The ease with which they move depends largely on the viscosity of the liquid (how easily it flows). Cooler liquids and those that are more densely packed (e.g., composition) are more viscous; the liquid has slower flow, and the atoms are less mobile.
Solidification into a crystal involves two key steps – nucleation and growth. For a polycrystalline solid to form, the nuclei (small clusters of atoms with a particular structure) must form, either on their own or with the help of other particles (impurities or those deliberately added, such as grain refiners). These nuclei form randomly and must reach a critical size to be stable.
Once these nuclei are stable, they will grow into grains. Crystal growth occurs by atoms in the liquid "fixing" onto the nuclei. They do so at particular sites which involve the least energy – these are called "preferred crystal directions." The grains will continue to grow until they collide with (impinge on) one another and all the liquid has solidified.
Pure metals vs. alloys
Pure metals solidify and melt at a single temperature, whereas alloys solidify and melt across a temperature range. These are bound by the solidus (the temperature at which the solid begins melting) and the liquidus (the temperature at which the liquid begins solidifying).
The solidus and liquidus temperatures vary with composition. During solidification, there is a temperature range at which the casting is partially solid and partially liquid – the "mushy" zone.
Casting and microstructure
As discussed, nearly all jewelry metals are crystalline. A crystalline object is usually polycrystalline – it is made up of many small crystals or "grains". The size and number of these grains depend on the processing history of the material.
Grain size varies from a few millimeters to hundredths of millimeters. Smaller grains usually lead to higher strength (and hardness) and toughness (higher resistance to cracking). It also reduces the risk of "orange peel" defects, etc. Fine grain size is desirable for jewelry.
Dendrites – what are they?
In alloys, casting often results in tree-like microstructures known as dendrites. The branches of the dendrite form along preferred crystal directions. As mentioned, atoms in the liquid can fix onto these directions with the least energy, so growth along these directions is fastest.
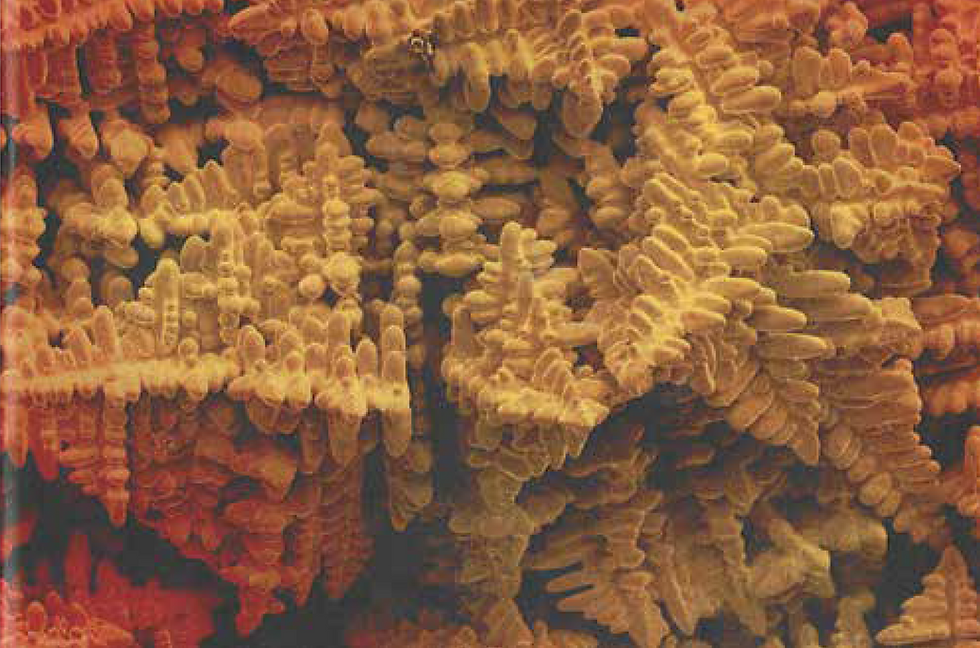
Dendrites occur in alloys because the composition of the liquid and solid during solidification may not be the same. These differing compositions mean that little bumps (perturbations) in the solid-liquid boundary during casting may be stable, and so the boundary isn't flat. This is known as constitutional undercooling – you can learn more in our explanation of defects or here.
Importance of cooling rate
Casting involves melting and the solidification of molten metal as it cools in the mold. When a metal solidifies, atoms, which are mobile and free to move in the liquid, fix onto growing crystals. Atoms in a crystal have fixed positions in the crystal lattice.
The cooling rate depends on the shape of the casting/mold and the mold material (higher conductivity means higher heat extraction and faster cooling). As the cooling rate increases, more nuclei will form (there is a larger undercooling below the liquidus temperature – the liquid is less stable than the solid). Once formed, all nuclei will grow at a similar rate. Ultimately, a higher cooling rate leads to a finer microstructure and smaller grain size.
Grain structures of ingots
Different grains in the polycrystalline ingot will have different orientations. When surrounded by liquid, the orientation of the nucleus (from which the grain grows) is random, but if we have nucleation near a mold wall, the grains may collectively be oriented similarly. This is known as "texture".
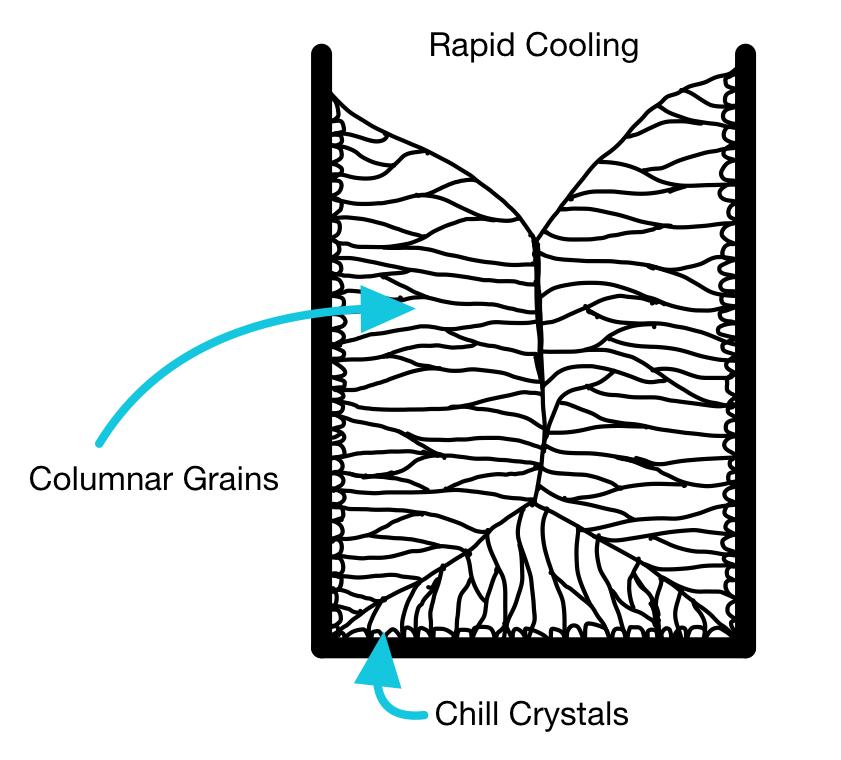
The grain structure of a whole ingot may be split into three key sections; next to the wall, an intermediate region, and the center of the ingot.
When the metal is poured into the mold, the metal near the mold wall will cool first. The wall acts as a favorable site for nucleation, so many nuclei form at once. A thin layer of fine grains is formed, known as the chill layer.

Some of these "chill" grains may be favorably oriented and so grow faster than others (the temperature gradient moving into the liquid aligns with the preferred growth direction). Long columnar grains grow inwards from the chill layer towards the center of the ingot.

Depending on the cooling rate, the center of the ingot can take different forms:
Using a metal mold, the columnar grains extend into the center of the ingot at high cooling rates. This can create issues in processing, known as "alligatoring".
At low cooling rates, using a ceramic mold e.g., investment casting, the cooling rate is low enough that nuclei can form in the liquid. This leads to "equiaxed" grain throughout the center of the casting.

Opmerkingen